|
|
ATP synthase FAQ
This list of Frequently Asked Questions (FAQ) on the ATP synthase is
written with the assumption that the reader has some background
knowledge in biochemistry, enzymology, and physical chemistry.
This is NOT a review article or something of that kind; there are no
references or credits, and no detailed description of the experiments
underlying each piece of information. If you are interested in getting
into details, just write me an e-mail (feniouk [at] atpsynthase.info)
and I will be glad to discuss any of the questions
below.
Recommended reading is added for some sections under
" "-sign. "-sign.
Table of Content
Correct name
Physiological role of ATP synthase
Differences between F-, A-, V-, P-, and E-ATPases
The architecture and subunit composition of ATP synthase
The reaction catalyzed
Thermodynamics of the ATP synthesis/hydrolysis
Driving force for ATP synthesis catalyzed by ATP synthase.
Rotary catalysis
Inhibitors of ATP synthase
Inhibitors of FO
Inhibitors of F1
Proton/ATP ratio
ATP synthase location
How many catalytic site does the enzyme have?
How fast is ATP synthase?
Proton translocation through FO
What is Beta DELSEED sequence?
Can I get an answer on a question not listed here?
Correct name
According to the IUBMB
Enzyme Nomenclature, the enzyme is called "ATP phosphohydrolase (H+-transporting)".
However, the name
"ATP synthase" reflects the primary function of the enzyme more
clearly and nowadays is most wide-spread.
The other name that was commonly used in the past is "H+-ATPase",
sometimes a more precise "FOF1
H+-ATPase". After the discovery of many other
types of ATP-driven proton pumps these old names are less used.
The other names that were used for ATP synthase are:
F1-ATPase
FOF1-ATPase
F-type ATPase or simply F-ATPase
H+-transporting ATPase
mitochondrial ATPase
coupling factors (F0, F1
and CF1)
chloroplast ATPase
bacterial Ca2+/Mg2+
ATPase
ATP synthase complex
Complex V (five)
Physiological role of ATP synthase
To make a long story short, the primary function of ATP synthase in most organisms is ATP synthesis.
Hence the name.
However, in some cases the reverse reaction, i.e. transmembrane proton pumping
powered by ATP hydrolysis is more important. A typical example: anaerobic bacteria produce ATP by
fermentation, and ATP synthase uses ATP to generate protonmotive force necessary for ion transport
and flagella motility.
Many bacteria can live both from fermentation and respiration or photosynthesis. In such case ATP synthase
functions in both ways.
An important issue is to control ATP-driven proton pumping activity of ATP synthase in order to avoid
wasteful ATP hydrolysis under conditions when no protonmotive force can be generated (e.g. leaky
damaged membrane, uncoupler present, etc.). In such case ATP hydrolysis becomes a problem,
because it can quickly exchaust the intecellular ATP pool. To avoid this situation,
all ATP synthases are equipped with regulatory mechanisms that suppress the ATPase
activity if no protonmotive force is present. The degree of ATP hydrolysis inhibition
depend on the organism. In plants (in chloroplasts), where it is necessary to preserve
ATP pool through the whole night, the inhibition is very strong: the enzyme hardly has any
ATPase activity. In contrast, in anaerobic bacteria where ATP synhase is the main
generator of protonmotive force, such inhibition is very weak. Mitochondrial ATP synthase is somewhere
inbetween.
Differences between F-, A-, V-, P-, and E-ATPases
- "F-type ATPase" is just another name for ATP synthase; letter "F"
comes
from "phosphorylation Factor".
F-ATPases are present in bacteria, mitochondria and chloroplasts. Their
major function in most cases is ATP synthesis at the expense of the transmembrane
electrochemical proton potential difference. In some bacteria,
however, the primary function of the enzyme is reversed: it
hydrolyzes ATP to generate this potential difference. In vitro F-type ATPases can
operate in both directions depending on the experimental conditions.
A few Na+-bacterial F-type ATPases are also found.
- A-type ATPases were found in Archaea,
their function is similar to that of F-type ATP synthase, but structurally they are very similar to V-type
ATPases (see below).
- V-type H+-ATPases were initially found in eukaryotic Vacuoles. Their primary
function is ATP-driven proton (or Na+) pumping to acidify the vacuol interior.
- P-type ATPases (sometimes named E1-E2 ATPases) pump a variety of
ions
across the membrane and are found in bacteria and in many
eukaryotic cell organelles.
- E-type ATPases (do not mix with E1-E2 ATPases!) is a family of
enzymes
that hydrolyze Extracellular
ATP (see Herbert
Zimmermann's Ecto-ATPase webpage for details)
F-, A-, and V-type ATPases are
multisubunit complexes, similar in terms of overall
architecture, and most probably have the same core catalytic mechanism. They
couple transmembrane proton (or Na+ in some F-ATPases) transport,
achieved by the rotation of a
certain subunits complex relative to the rest of the enzyme, with ATP
hydrolysis (or synthesis in A- and F-ATPases).
The common features for them are: "mushroom" shape, hexameric
hydrophilic catalytic domain of Alpha 3 Beta 3
- type with Gamma subunit inside it. The catalytic act performed by
those enzymes does not include a phosphorylated enzyme
intermediate.
The proton-translocating portion
of those enzymes is composed of a ring-shaped subunit oligomer (c-subunit
oligomer in case of F-type ATPases); each
subunit bears a critically important
carboxyl group approximately in the middle of its second transmembrane
helix. This carboxyl group is directly involved in
proton translocation.
P-type ATPases are quite a different family of
ion-translocating ATP-driven pumps. Most of them are also multisubunit
membrane proteins; one large f performs both ATP
hydrolysis and ion pumping. There are many different subfamilies of
P-type ATPases, usually classified according to the ion they
transport. H+, Na+, K+, Mg2+, Ca2+, Ag+ and
Ag2+, Zn2+,
Co2+, Pb2+,
Ni2+, Cd2+, Cu+ and Cu2+ pumping P-ATPase are
described.
During ATP hydrolysis by a P-ATPase
at a certain stage of catalytic cycle the phosphate is transferred to
one of the Asp residues of the enzyme. There is no evidence (neither
structural nor functional) for rotary catalysis in P-type ATPases.
Typical examples of such enzymes are yeast plasma membrane H+
ATPase, K+/Na+ membrane
ATPase, Ca2+ membrane
ATPase.
 |
1) Pedersen, P. L., and
Carafoli, E. (1987) Ion motive ATPases. I. Ubiquity, properties, and
significance to cell function. Trends Biochem. Sci. 4:
146-150.
2) P-type ATPase
Database (By Kristian B. Alexsen, Swiss Institute of Bioinformatics)
3) Kawasaki-Nishi S, Nishi T, Forgac M. (2003 ) Proton translocation
driven by ATP hydrolysis in V-ATPases.
FEBS Lett. 545(1): 76-85.
4) Perzov N, Padler-Karavani V, Nelson H, Nelson N. (2001) Features of
V-ATPases that distinguish them from F-ATPases. FEBS Lett. 504(3): 223-8.
|
The architecture and subunit composition of ATP synthase
ATP synthase is a large mushroom-shaped asymmetric protein complex. The
simplest bacterial enzyme (see the cartoon below) is composed of 8
subunit types, of
which 5 form the catalytic hydrophilic F1-portion (the "cap"
of the mushroom). These subunits are named by Greek letters (Alpha,
Beta, Gamma, Delta and Epsilon) in accordance with their molecular
weight. The proton translocating FO portion is composed of
subunits of 3 types named a, b and c.

The catalytic portion of ATP synthase (F1) is formed by
Alpha 3
Beta 3 hexamer with Gamma subunit inside it and Epsilon
attached to the Gamma. Subunit Delta is bound to the "top" of the
hexamer and to
subunits b.
The hydrophobic transmembrane segment of subunit b is in contact
with subunit a.
Subunits Gamma and Epsilon of the catalytic domain are bound to the
ring-shaped oligomer of c-subunits.
Proton translocation take place at the interface of subunits a and c.
The stoichiometry of the subunits is:
F1
|
FO
|
Alpha
|
3
|
a
|
1
|
Beta
|
3 |
b
|
2
|
Gamma
|
1
|
c
|
10-15(?)
|
Delta
|
1
|
|
|
Epsilon
|
1
|
|
|
Chloroplast ATP synthase and the enzyme from some photosynthetic
bacteria
have 2 different, although similar, b-type
subunits in the proton
translocating FO p
ortion, namely b and b', one copy of
each.
High homology is found for most of the ATP synthase subunits from
different
bacteria and chloroplasts.
Mitochondrial enzyme is much more complex; 17 different types of
subunits are described at the moment. Some of these subunits have
high homology to bacterial and
chloroplast counterparts, especially subunits Alpha, Beta and Gamma in
the F1 portion and subunits a and c in the FO
portion. Many subunits are unique for the mitochondrial enzyme (see Subunit Nomenclature Table
for details).
However, the catalytic and proton translocating "core" of the enzyme is
still highly homological to that of bacterial and chloroplast ATP
synthase. The overall topology of the enzyme is also quite similar.
The reaction catalyzed
ATP synthase catalyzes ATP synthesis/hydrolysis coupled to
transmembrane proton transfer.
In case of synthesis the energy input comes
from protonic flux through FO downhill the transmembrane
electrochemical proton potential difference ( ).
In case of hydrolysis the enzyme functions as an ATP-driven proton pump
and generates ).
In case of hydrolysis the enzyme functions as an ATP-driven proton pump
and generates  . .
The equation of the reaction catalyzed is
ADP3- + Pi2- + nH+P <=> ATP4- + H2O
+ (n-1)H+N
( pH > 7.2 )
The "P" and "N" indices denote the positively and the negatively charged sides of the
coupling membrane.
The pH value is important: the pK value for Pi2- + H+
<=> Pi- is 7.2, while the
corresponding pK values for phosphate in ADP and ATP are close to 6.9.
This means that in the pH interval of 6.9-7.2 the prevailing
reaction will not include trapping of protons:
ADP3- + Pi- + nH+P
<=> ATP4- + H2O
+ nH+N
( pH 6.9-7.2 )
However, below pH = 6.9, the prevailing reaction is again accompanied
by proton
trapping:
ADP2- + Pi- + nH+P <=> ATP3- + H2O
+ (n-1)H+N
( pH < 6.9 )
Thermodynamics of the ATP synthesis/hydrolysis
Traditionally the thermodynamics of ATP synthesis/hydrolysis is
described for the hydrolysis reaction:
ATP4- + H2O <=> ADP3- + Pi2- + H+
( pH > 7.2 )
"Physical Chemistry"
(P.W.Atkins, 2nd edition) gives a value of -30 kJ mol-1 (-7.16 kcal/mol) at 37oC
as a "biological" standard Gibbs
free energy change ( o´) for this
reaction. This is a reasonable estimate, for figures from -28 to -36 kJ
mol-1
can be found in literature, the most popular being -30.6 kJ mol-1
(-7.3 kcal/mol). o´) for this
reaction. This is a reasonable estimate, for figures from -28 to -36 kJ
mol-1
can be found in literature, the most popular being -30.6 kJ mol-1
(-7.3 kcal/mol).
The standard Gibbs
free energy change,  o, is the total amount
of energy which is either used up or released during a chemical
reaction under standard
conditions when the chemical activities of all the reactants is
equal to 1. In case of reactions in aqueous solutions the activities
are usually substituted by concentrations (i.e. 1 M); the activity of
water itself is taken as 1. "Biological" standard Gibbs
free energy change, o, is the total amount
of energy which is either used up or released during a chemical
reaction under standard
conditions when the chemical activities of all the reactants is
equal to 1. In case of reactions in aqueous solutions the activities
are usually substituted by concentrations (i.e. 1 M); the activity of
water itself is taken as 1. "Biological" standard Gibbs
free energy change,  o´, is a similar
parameter, but is defined at pH 7, i.e. the concentration of H+
is not 1 M, but 10-7M. It is more practical and convenient,
for most biological reactions take place at physiological pH. o´, is a similar
parameter, but is defined at pH 7, i.e. the concentration of H+
is not 1 M, but 10-7M. It is more practical and convenient,
for most biological reactions take place at physiological pH.
A very important, and sometimes ignored point, is that  o´ is not the amount of energy available
to drive other, endothermic reactions in
the cell, because the conditions in the cell are not standard
(see the definition above). The actual Gibbs energy change is o´ is not the amount of energy available
to drive other, endothermic reactions in
the cell, because the conditions in the cell are not standard
(see the definition above). The actual Gibbs energy change is
 = =  o'
+ 2.3 RT log [CADP
CPi (CH+ / 10-7) / CATP ], o'
+ 2.3 RT log [CADP
CPi (CH+ / 10-7) / CATP ],
where CADP,
CPi, CH+,
and CATP are the actual concentrations of
the corresponding reactants, R is the molar gas constant
(8.314 J mol-1K-1), and
T is the temperature in Kelvins. To
make this point clear, let us consider the following example with the
arbitrary values that are close to the real intracellular
concentrations:
| CATP |
2 x 10-3 M-1 |
| CADP |
2 x 10-4 M-1 |
| CPi |
10-2 M-1
|
| CH+ |
5 x 10-8 M-1(pH
approx. 7.3)
|
The Gibbs energy change under such conditions (temperature 310oK,
or 37oC) will be
 = =  o'
+ 2.3 RT log ( CADP
CPi CH+ / CATP )
= -30 - 19.6 = - 49.6 kJ mol-1 o'
+ 2.3 RT log ( CADP
CPi CH+ / CATP )
= -30 - 19.6 = - 49.6 kJ mol-1
This figure, calculated from the actual concentrations of the
reaction components, reflects the energy available as a driving force
for any other process coupled to ATP hydrolysis under given conditions.
It follows that the same 49.6 kJ mol-1 must be provided by
the proton transport across the membrane down the electrochemical
gradient to maintain such a high ATP/ADP ratio. If we assume that 3
protons are transported per each ATP molecule synthesized, a
transmembrane H+ electrochemical gradient of 49.6 / 3
= 16.5 kJ mol-1
(i.e., protonmotive
force of 171 mV) is necessary.
The conclusion from the example above is:
The energy provided by ATP hydrolysis is not fixed (as well as the
energy necessary to synthesize ATP). In
first approximation it depends on the concentrations of ADP, ATP, Pi
and on the pH. This energy increases logarithmically upon decrease in
ADP and Pi concentration and upon increase in ATP or H+
concentration (= decreases linearly with increase in pH). The graphs below illustrate this point, showing
change in the  upon the change in the
concentration
of one reactant (x axis),
assuming that the concentrations of other reactants are kept constant
at values used in the example above (red dots indicate the upon the change in the
concentration
of one reactant (x axis),
assuming that the concentrations of other reactants are kept constant
at values used in the example above (red dots indicate the  calculated in this example).
calculated in this example).

To close up this section, I would like to note that although the
thermodynamics of the ATP synthesis described here might seem rather
complex, it is actually much more complex. One point neglected here was
the different ADP and ATP protonation states (see
above), the other is that the actual substrates in the reaction
catalyzed by ATP synthase are not pure nucleotides, but their magnesium
complexes. However, as the magnesium concentration in the living cell
is relatively high and the pH is usually above 7.2, so the description
given is still applicable for thermodynamic estimates.
 |
1) Nicholls, D. G. and S.
J. Ferguson. Bioenergetics 2,
London:Academic Press, 1992.
2) Any edition of "Physical Chemistry"
by P. Atkins
|
Driving force for ATP synthesis catalyzed by ATP synthase.
ATP synthesis catalyzed by ATP synthase is powered by
the transmembrane electrochemical proton potential difference,  composed of two
components: the chemical and the
electrical one. The more protons are on one side of a membrane relative
to
the other, the higher is the driving force for a proton to cross the
membrane. As proton is a charged particle, its movement is also
influenced by electrical field: transmembrane electrical potential
difference will drive protons from positively charged side to
the negatively charged one. composed of two
components: the chemical and the
electrical one. The more protons are on one side of a membrane relative
to
the other, the higher is the driving force for a proton to cross the
membrane. As proton is a charged particle, its movement is also
influenced by electrical field: transmembrane electrical potential
difference will drive protons from positively charged side to
the negatively charged one.

A water mill is a good analogy: the difference between the water levels
before and after the dam provides potential energy; downhill water flow
rotates the
wheel; the rotation is used to perform some work (ATP synthesis in our
case).

Quantitatively  is measured in Joules per mole (J mol-1) and is
defined as:
is measured in Joules per mole (J mol-1) and is
defined as:
 =
-F
=
-F + 2.3
RT (pHP - pHN), + 2.3
RT (pHP - pHN),
where the "P" and "N" indices denote the positively and the negatively charged sides of the
coupling membrane; F is Faraday constant
(96 485
C mol-1); R is the molar gas constant
(8.314 J mol-1K-1),
T is the temperature in Kelvins, and  is the
transmembrane electrical potential difference in
volts. The value of is the
transmembrane electrical potential difference in
volts. The value of  tells, how much energy is required (or is released, depending on the
direction of the transmembrane proton flow) to move 1 mol of protons
across the membrane.
tells, how much energy is required (or is released, depending on the
direction of the transmembrane proton flow) to move 1 mol of protons
across the membrane.
It is often more convenient to use not  , but protonmotive force (pmf): , but protonmotive force (pmf):
pmf = -  /
F = /
F =  -
2.3
RT/F (pHP - pHN) -
2.3
RT/F (pHP - pHN)
At room temperature (25oC) the protonmotive force (in
millivolts, as well as  )
is: )
is:
pmf =  - 59 (pHP
- pHN) - 59 (pHP
- pHN)
In the absence of transmembrane pH difference pmf equals the transmembrane
electrical potential difference and can be directly measured by several
experimental techniques (i.e. permeate ion distribution,
potential-sensitive dyes, electrochromic carotenoid bandshift, etc.).
Each pH unit of the transmembrane pH gradient corresponds to 59 mV
of pmf.
For most biological membranes engaged in ATP synthesis the pmf value lies between 120 and 200
mV ( between 11.6 and
19.3 kJ mol-1). between 11.6 and
19.3 kJ mol-1).
 |
1) Nicholls, D. G. and S.
J. Ferguson. Bioenergetics 2,
London:Academic Press, 1992.
2) A Lecture on
Electrochemical potential by Prof. A.R. Crofts
3)Cramer, W.A. and D.B. Knaff. Energy
Transduction in Biological Membranes: A Textbook of Bioenergetics, Springer-Verlag
New York/Berlin/London
|
Rotary catalysis
The catalytic mechanism of ATP synthase
most probably involves rotation of Gamma subunit together with subunit
Epsilon and c-subunit
oligomer relative to the rest of the enzyme. Such rotation was
experimentally shown for ATP hydrolysis uncoupled to proton
translocation. Moreover, recent experiments revealed, that if Gamma
subunit is mechanically forced into rotation, ATP synthesis takes place
even without proton-translocating FO-portion.
It seems most probable that such rotation takes place in vivo. However, there is no
direct experimental evidence for such rotary mechanism in the intact
enzyme under physiological conditions.
The proposed mechanism is the following:
- Driven by the protonmotive
force, protons are transferred through the FO portion of
the enzyme. This transfer drives the rotation of the c-subunit
oligomer ring relative to the a and b subunits (see here for details).
- The rotation is passed to Gamma and Epsilon subunits that
are bound to the c-subunit
oligomer ring. The rotation of asymmetric Gamma subunit mechanically
causes conformational changes in Alpha 3 Beta 3
-hexamer. Each 120 degrees of the Gamma subunit rotation
forces one of 3 catalytic sites located at Alpha-Beta interface into an
opened conformation. Freshly synthesized ATP molecule is released, and
phosphate and ADP are bound instead. High affinity of the opened site
to
phosphate impairs rebinding of ATP and favours ADP binding.
- Rotation goes further, Gamma subunit turns another 120 degrees
forcing the next site into the opened conformation, and the ADP and
phosphate bound to the previous opened site are occluded and ATP
synthesis takes place. The ATP molecule formed is released when the
Gamma subunit makes one 360 degrees turn and once again opens the site.
 |
1) W. Junge, H. Lill, and
S. Engelbrecht. (1997) ATP synthase:
an
electrochemical transducer with rotatory mechanics. Trends Biochem.Sci. 22
(11):420-423, .
2) H. Wang and G. Oster. (1998) Energy
transduction in the F1 motor of ATP synthase. Nature 396 (6708):279-282.
3) Weber, J., and Senior, A. E. (2003) ATP
synthesis driven by proton transport in F1FO-ATP
synthase.
FEBS Lett. 545(1): 61-70.
4) Nice movies at http://nature.berkeley.edu/~hongwang/Project/ATP_synthase/
|
Inhibitors of ATP synthase
ATP synthase activity is specifically inhibited by several compounds
(both organic and inorganic). Most of these inhibitors are very toxic, so great care
and appropriate safety precautions are essential when working with them (it is not very surprising that
we get unhappy when OUR ATP synthase is blocked!).
Most inhibitors are specific for either proton-translocating FO-portion, or hydrophilic
F1-portion, so the section below is divided accordingly.
Inhibitors of FO
Oligomycin

Oligomycin A
Oligomycin is the inhibitor that gave the name "FO" to the membrane-embedded portion of ATP synthase.
The subscript letter "O" in FO(not zero!) comes from Oligomycin sensitivity of this hydrophobic
phosphorylation Factor in mitochondria.
Oligomycin binds on the
interface of subunit a and c-ring oligomer and blocks the rotary
proton translocation in FO. If the enzyme is well-coupled, the activity of F1
is also blocked. Because of the latter phenomenon, a subunit of mitochondrial F1-portion
that connects F1 with FO was named Oligomycin-Sensitivity Conferring Protein (OSCP).
This subunit is essential for good coupling between F1 and FO
and makes the ATPase activity of F1 sensitive to FO inhibitor
oligomycin, hence the name.
Oligomycin is specific for mitochondrial ATP synthase and in micromolar concentrations
effectively blocks proton transport through FO. This inhibitor also works in
some bacterial enzymes that show high
similarity to mitochondrial ATP synthase, e.g. enzyme from purple bacterium Rhodobacter capsulatus.
But ATP synthase from chloroplasts and from most bacteria (including Escherichia coli)
has low sensitivity to oligomycin.
It should also be noted that oligomycin in high concentrations also affects the activity of mitochondrial F1.
DCCD

DCCD
DCCD (abbreviation for Dicyclohexylcarbodiimide; also known as DCC, as N,N'-dicyclohexylcarbodiimide,
as Bis(cyclohexyl)carbodiimide, and as 1,3-dicyclohexylcarbodiimide) is a small organic molecule that
can covalently modify protonated carboxyl groups. When added to ATP synthase at pH above 8,
DCCD almost exclusively reacts with the carboxyl group of the conserved acidic amino acid
residue of subunit c (that is why subunit c is sometimes called "DCCD-binding protein"). that has elevated pK and can therefore be protonated at such a
high pH. Modification of the carboxyl group in a single c-subunit is enough to render
the whole c-ring oligomer inactive. Because DCCD covalently binds to c-subunit,
this inhibition is irreversible.
The carboxyl group of the conserved amino acid residue in subunit c-subunit is present in
all ATP synthases known so far. So DCCD is a universal inhibitor that can FO function
in bacterial, mitochondrial and chloroplast enzymes. Moreover, V- and A-type proton-transporting ATPases
are also sensitive to DCCD for the same reason. Sodium-transporting ATP synthases are also effectively inhibited by DCCD.
At lower pH (<7) DCCD modifies several carboxyl groups in F1 and inactivates it. So this compound can
be considered as an inhibitor of both FO and F1. However, inhibition of FO
is highly specific, well-defined, and requires much lower DCCD concentration so usually this
inhibitor is used as FO-specific.
Venturicidin
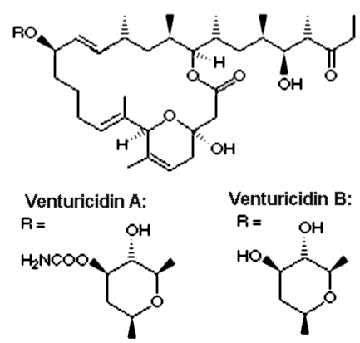
Venturicidin
The macrolide antibiotic venturicidin (also known as Aabomycin) isolated from a Streptomyces sp. was originally
described as an antifungal agent.
Later it was found that venturicidin is a potent inhibitor of ATP synthase that specifically
blocks proton translocation through FO. Like oligomycin, it binds on the interface of
subunit a and c-ring oligomer. However, venturicidin specificity
is not limited to mitochondrial ATP synthase, and it is effectively inhibiting bacterial and chloroplast
enzymes. Na+-translocating ATP synthases are also strongly inhibited with venturicidin.
If the coupling between FO and F1 is good, venturicidin also blocks the
activity of F1. So this inhibitor is a good choice for quick test of the coupling efficiency.
Its important advantages over DCCD are quick effect and ease of use. Unlike DCCD, venturicidin can be stored
as a concentrated stock solution for a long time without loss of inhibitory power.
The affinity of FO to venturicidin is very high. In Rhodobacter capsulatus
ATP synthase half-maximal inhibition was observed at 2-5 nM venturicidin concentration.
Inhibitors of F1
Azide
Azide selectively inhibits ATPase activity of ATP synthase, leaving its ATP synthesis activity unaffected. It is demonstrated in
mitochondrial F1 that azide binds together with MgADP (interacting with its beta-phosphate) in a catalytic site, and presumably prevents ADP
release from this site. However, rotation of subunit gamma forced by sufficiently high pmf or by external force
can expell the occluded ADP from the catalytic site, bringing the enzyme to active ATP synthesis.
Tentoxin
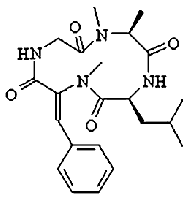
Tentoxin
Tentoxin is a phytotoxin produced by fungi of the Alternaria species. It
specifically inhibits the ATPase activity of some chloroplast ATP synthases; it has no effect on bacterial and
mitochondrial enzyme. Moreover, some chloroplast ATP synthases are also tentoxin-resistant.
Tentoxin binds at the cleft between Alpha and Beta subunits close to
the N-terminal beta-barrel crown of F1. At small concentration (about 1-10uM) tentoxin
inhibits ATP hydrolysis, while at higher concentrations the inhibition is relieved.
The binding site of tentoxin was determined by X-ray analysis of chloroplast F1 crystallized
in the presence of the inhibitor.
Efrapeptin
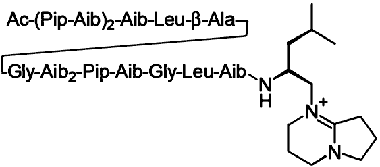
Efrapeptin C
Efrapeptin (also known as A 23871 or A23871) is a common name for a
group of small peptides antibiotics that can bind inside F1 with high affinity and inhibit
both ATP synthesis and hydrolysis.
The binding site of efrapeptin was determined by X-ray analysis of
the bovine mitochondrial F1 crystallized
in the presence of the inhibitor.
It is likely that efrapeptin fixes subunit Gamma inside F1
and block the rotation of this subunit.
Efrapeptins are potent inhibitors for mitochondrial ATP synthase and for some bacterial enzymes.
The inhibitory effect was first noticed in chromatophores of purple
bacterium Rhodospirillum rubrum. Chloroplast ATP synthase is only mildly sensitive to efrapeptin.
Fluoro-aluminate (AlF4)
Fluoro-aluminate based inhibitors mimic the transitional state of ATP gamma-phosphate.
They bind together with ADP in catalytic sites and freeze the enzyme in a
conformation that presumably reflects an intermediate step of ATP hydrolysis\synthesis.
Proton/ATP ratio
From the early experiments with mitochondria the H+/ATP
ratio for ATP synthesis was estimated as 3. However, for chloroplast
enzyme the figure of 4 was found more probable. From the thermodynamic
considerations less than 3 protons pro ATP is hardly feasible, for the
energy required for ATP synthesis under physiological conditions is
about 50 kJ mol-1 (~520
meV), so at
physiological protonmotive force values in
the range of 120-200 mV at least 3 protons should be transferred to get
the energy necessary.
There is no convincing evidence or arguments that this ratio
should be a whole number.
This ratio is expected to depend on the number of c-subunits in
the FO: as there are 3 catalytic sites on
the enzyme and
it is most possible that ATP synthesis is driven by a rotary mechanism,
H+/ATP
= (number of c-subunits)
/ 3
But here the problem is that the experimentally determined numbers of
the c-subunits
in ATP synthases from different organisms are 10, 11, 14, and 15, suggesting ratios
of 3.33, 3.67, 4.67 and 5,
respectively. It is also possible that c-subunit
stoichiometry varies depending on the situation in the cell.
ATP synthase location
ATP synthase is found in bacteria, mitochondria and chloroplasts. In
bacteria it is located in the cell membrane with the bulky hydrophilic
catalytic F1 portion sticking into cytoplasm. The
orientation is quite easy to remember, for the bacterium need ATP to be
synthesized inside the cell, not outside. With the proton flow it is
less easy; I found it helpful to think that protons always go
“along” with ATP: during ATP synthesis they enter the bacterial cell
(more ATP inside, more protons inside), and during ATP hydrolysis they
leave the cell and go into the outer medium (less ATP inside, less
protons inside).
In mitochondria ATP synthase is located in the inner membrane, the
hydrophilic catalytic F1 portion is sticking into matrix. In
a way a mitochondrion is a bacterium
“swallowed” by the eukaryotic cell: then the inner mitochondrial
membrane corresponds to the bacterial cell membrane.
In chloroplasts the enzyme is located in the thylakoid membrane; F1
portion is sticking into the stroma.

How many catalytic site does the enzyme have?
|
The answer is three. Not five.
However, the total number of the nucleotide-binding
sites is six, three of them being non-catalytic. Each
site is located on the interface between subunits Alpha and Beta.
Larger part of each catalytic site is composed from amino acid residues
of the
respective Beta-subunit, while each non-catalytic site is situated
mostly on the respective Alpha subunit. The role of the non-catalytic
sites is probably regulatory, they are not necessary for the catalysis. Occupation of the non-catalytic sites
by nucleotides was shown to increase the enzyme activity. It is also
possible that binding of nucleotides to the non-catalytic sites facilitate the enzyme assembly in the cell.
There is strong evidence that in bacteria of Bacillus genera
the Epsilon subunit also can bind one nucleotide. So in Bacillus ATP synthase there are 7 nucleotide binding sites!
|

|
How fast is ATP synthase?
For simplicity let us leave aside the more "biochemical", but less
understandable values of "micromoles of ATP per minute per mg protein"
and
discuss the number of ATP molecules synthesized (or hydrolyzed) by one
ATP synthase in one second.
Maximal rates over 100 s-1 were reported for bacterial,
mitochondrial and chloroplast enzymes for ATP synthesis. ATP hydrolysis
rates is a less clear issue, for the coupled enzyme in small membrane
vesicles (most commonly used experimental system) quickly builds up
relatively high protonmotive force that acts as a back pressure and
stops the hydrolysis. For uncoupled or solubilized enzyme rates over
100 s-1 were also reported.
In the living cell the enzyme most probably operates below the maximal
possible rate, making tens of ATP molecules per second.
 |
1) C. Etzold, G.
Deckers-Hebestreit, and K. Altendorf. (1997) Turnover number of Escherichia coli FOF1- ATP synthase for ATP synthesis in
membrane vesicles. Eur.J.Biochem.
243 (1-2):336-343.
2) R. L. Cross, C. Grubmeyer, and H. S. Penefsky. (1982) Mechanism of
ATP hydrolysis by beef heart mitochondrial ATPase. Rate enhancements
resulting from cooperative interactions between multiple catalytic
sites. J.Biol.Chem.
257:12101-12105.
3) U. Junesch and P. Gräber. (1985) The rate of ATP synthesis as a function of
Delta pH in normal and dithiothreitol-modified chloroplasts. Biochim.Biophys.Acta 809:429-434.
|
Proton translocation through FO
Although the Fo portion of the ATP synthase is often referred to as
"proton(ic) channel", it is NOT a channel. It differs significantly
from "real" proton channels (e.g. gramicidin,
M2 from influenza
virus, etc.). The most important distinction is that when being in
conducting state, a membrane channel does not require conformational
changes for proton translocation, while FO portion of ATP
synthase does. The transfer rate is also too slow for a channel: at
voltage of 100 mV textbooks give a rate of about 106
ions per second for an ion channel, more than 100-fold higher than the maximal
corresponding values reported for FO portion. So the latter
is a typical example of a proton transporter (the ability to operate as
a pump is further confirming it - no channel can do that).
However, the term "proton channels" is often used for certain regions
in the membrane proteins that are involved in proton translocation
(e.g. proton channels in the cytochrome oxidase, or proton entrance channel
in bacteriorhodopsin). As they never cross the entire membrane,
they are sometimes called "proton half-channels".
The proton-translocating region of ATP synthase is formed by subunit a and c-subunit
oligomer. There are two certain amino acid residues that are
critically important for proton translocation. The first is an acidic residue
(mostly Glu, in some organisms Asp) in the middle of the second
transmembrane alpha-helix of subunit c. The second is
an Arg at the
last but one transmembrane helix of subunit a. Almost all
mutations in
those two residues result in a complete loss of activity. Several other
important hydrophilic amino acid residues are located on subunit a,
but their substitution leads only to a partial loss of activity.
The currently favored hypothesis of proton transport through ATP
synthase is
based on the stochastic rotary mechanism. It is presumed, that the
conserved acidic residue on the c-subunit can be
deprotonated (i.e. negatively charged) only when facing the
protein-protein interface between a and c subunits,
because it is energetically unfavorable to expose a charge into
hydrophobic lipid bilayer.
Proton enters through one half-channel, binds to the unprotonated,
negatively charged carboxyl group of the c-subunit
conserved Glu (or Asp). The latter becomes electrically neutral and can
now enter the hydrophobic lipid phase. As soon as it does, another c-subunit with
protonated Glu (Asp) comes from the lipid phase into protein-protein
interface area from the other side and releases its proton through the
other half-channel. Carrying now a negative charge, it cannot go back,
but can go one position forward and accept another proton from the
first half-channel. The cycle is completed. Click here
for an animated cartoon illustrating the mechanism above, or
download a much nicer (and therefore much larger) movie
from Prof. Junge's webpage!
What is Beta DELSEED sequence?
Beta DELSEED region is a part of subunit Beta that has amino acid sequence of
-Asp-Glu-Leu-Ser-Glu-Glu-Asp- (hence the name: in single-letter amino acid code it is DELSEED).
This fragment is highly conserved in all ATP synthases. However, its role is not completely clear.
In bacterial ATP synthase from thermophilic Bacillus PS3 it was demonstrated that this region
is essential neither for ATP hydrolysis nor for ATP-driven rotation of subunit Gamma in Alpha3-Beta3 complex,
but plays a role in the inhibitory action of subunit Epsilon. It is likely that in Bacillus PS3
the negatively charged Asp and Glu residues interact with positively charged Lys and Arg in the
C-terminal domain of Epsilon, and block hydrolysis.
It is probable that the same mechanism works in ATP synthase from
other bacteria and in chloroplast enzyme. In mitochondrial ATP synthase such mechanism is unlikely,
because subunit Delta (mitochondrial homologue of bacterial epsilon)
lacks the important positive charges in its C-terminal domain.
 |
1)Hara, K.Y., Kato-Yamada, Y., Kikuchi, Y., Hisabori, T., and Yoshida, M. (2001)
The role of the betaDELSEED motif of F1-ATPase: propagation of the inhibitory effect of the epsilon subunit.
J. Biol. Chem. 276(26):23969-23973.
2)Feniouk, B.A., Suzuki, T., and Yoshida, M.(2006)
The role of subunit epsilon in the catalysis and regulation of FOF1-ATP synthase.
Biochim Biophys Acta. 1757(5-6):326-338.
|
Can I get an answer on a question not listed here?
Sure. E-mail me:
feniouk [at] atpsynthase.info
|
|